Cultured blood and gene therapy for patients with anemia
NewsIn the future, Sanquin wants to grow red blood cells on a large scale for chronic transfusion patients, such as those with sickle cell disease or Diamond-Blackfan anemia syndrome. To do so, the researchers will use so-called induced pluripotent stem cells. A next step is to genetically repair such cells, in this case derived from the patient itself, to cure the disease.
Induced pluripotent stem cells (iPSC) can be made from any body cell, according to Eszter Varga. She is a stem cell biologist, senior postdoc and head of Sanquin's iPSC facility. She herself makes iPS cells from white blood cells derived from donor blood. `We add certain proteins - so-called transcription factors - so that these blood cells reprogram themselves to a stage as if they were embryonic stem cells. You can culture these stem cells in any direction. We turn them into red blood cells. This is already possible in the laboratory, which itself is unique, but we want to considerably scale up this production in bioreactors. With the cultured blood we hope to treat people who are dependent on blood transfusions but for whom it is difficult or impossible to find matching blood, because of their very specific blood group profiles.'
The TRACER project
Within the Dutch project TRACER, in which not only the blood institute Sanquin but also several universities participate, the iPS cells are specifically used for research into sickle cell disease and Diamond-Blackfan anemia syndrome. These are hereditary forms of anemia. Sickle cell disease, rare in the Netherlands but the most common hereditary disorder worldwide, is caused by deformed red blood cells, that also have a very short life span. In the rare Diamond-Blackfan anemia syndrome, the bone marrow does not produce enough red blood cells. Both diseases can be accompanied by fatigue, shortness of breath and palpitations. Sickle cell disease also carries the risks of extreme pain attacks, and blockages in the veins may cause strokes and organ damage. Varga: `Within TRACER, which stands for TReating hereditary Anemias through stem CEll Research, we use iPS cells from patients to create disease models to study both forms of anemia in the laboratory. With these models, it is also possible to test new therapies. Because very few patients in the Netherlands have these diseases, this is a welcome alternative for research.
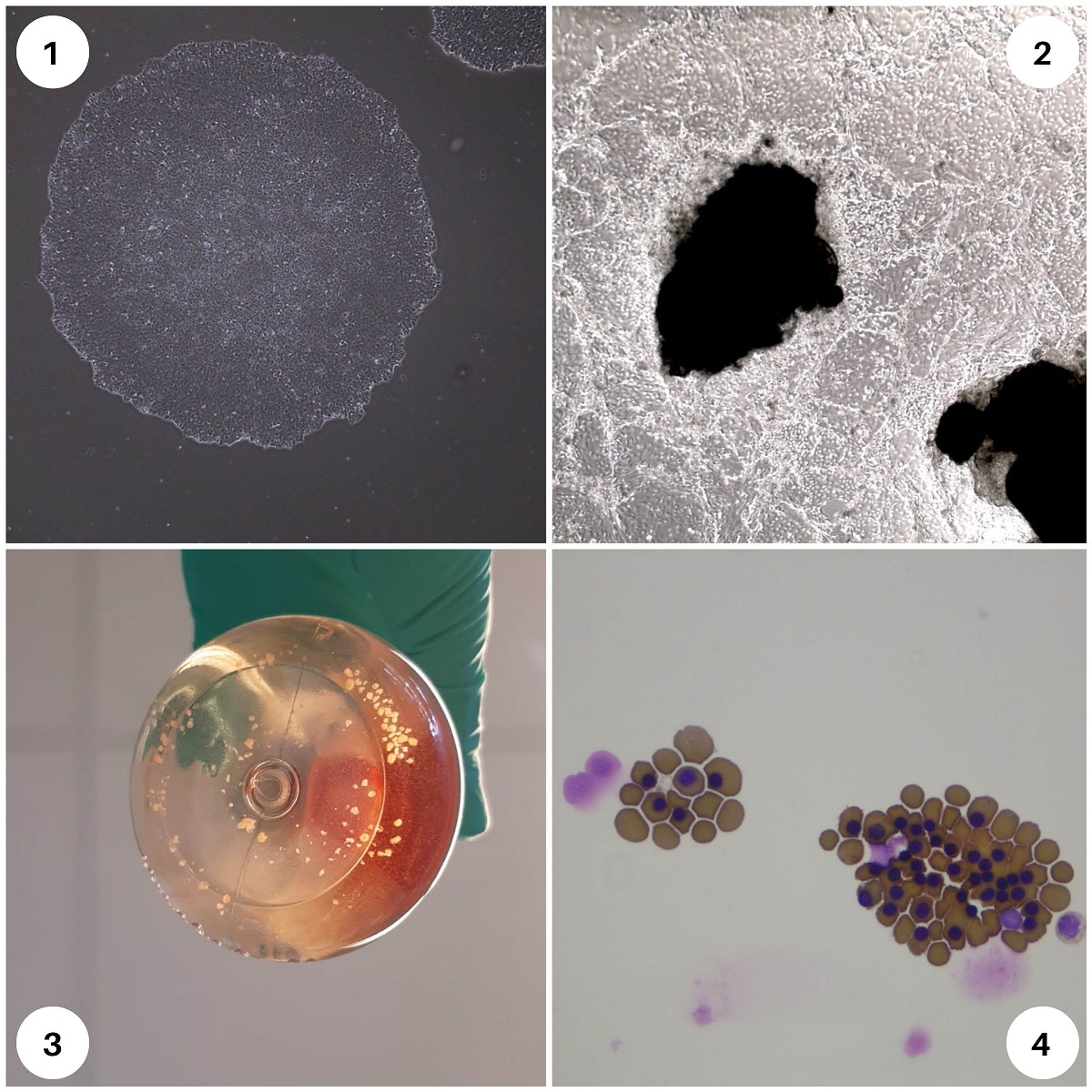 1: iPSC colonies, 2, 3: organoids, 4: red blood cells
1: iPSC colonies, 2, 3: organoids, 4: red blood cells
No rejection
TRACER also has another goal. The researchers also want to use the iPS cells to investigate gene therapy. The plan is to make iPS cells from a patient's white blood cells and then switch off or repair the pathogenic genes using the CRISPR-Cas gene editing-technique. By allowing these patient-grown iPS cells to develop into bone marrow cells, it is possible to return these healthy cells to the patient. As a major advantage, Varga mentions that the match is then perfect because it is the patient's own material. You don't have to be afraid of rejection reactions, a small risk that does exist with donor material. That immune reaction can even be fatal. As mentioned, we can already produce red blood cells from iPS cells in the lab, but for bone marrow cells to use for transplantations we are not yet successful. We are now making a step-by-step plan for that, which in particular my colleagues are working on. In the future, combining bone marrow stem cell production from iPSC with genetic repair of the disease will make blood transfusions during patient’s lifetimes no longer needed.'
Social and ethical issues
For transfusions of cultured blood and genetically modified red blood and bone marrow cells to be done appropriately in the future, Louisanne van Hooff is working on the ethical and social issues surrounding these treatments. She is a medical anthropologist at Sanquin and, like Varga, closely involved with TRACER. `Not everyone is open to cultured blood and gene therapy. Many people are reluctant to tinker with our DNA. That is why it is very important to talk to the people involved.'' Van Hooff interviews not only patients but also their caregivers and family members. `We want to know what they think of these therapies and what their wishes are. We also look at what cultural values underlie it. With sickle cell disease in the Netherlands, for example, you are often dealing with people who have ancestors from sub-Saharan Africa, where malaria is endemic. That arises from the evolutionary advantage of sickle cell trait against malaria. For instance people from Suriname and the Caribbean. We are also looking at which healthcare organizations patients can turn to.' A tricky subject, according to Van Hooff, is the accessibility of treatments. `These are still extremely expensive. Several million euros per patient. It should not be the case that soon only wealthy people will be able to make use of it. We have to prevent that.'
Info:
Wednesday, June 19, is World Sickle Cell Day. On that day, an exhibition starts at museum OSCAM in Amsterdam Zuidoost, where the disease and possible therapies are the focus. There is also attention to the lack of donors with ancestors in sub-Saharan Africa.
