
Cytokine analysis - ELIspot
The ELIspot assay is a robust method to detect the number of antigen-specific responses in peripheral blood. For B cells, CD4+ T cells and CD8+ T cells, the ELIspot can provide information about the amount of cytokine-producing cells, while for B cells, we can also determine the specific isotype of produced antibodies.
For the simultaneous measurement of 3 cytokines or 3 types of antibody isotypes, a Fluorospot analysis can be performed. This provides information about e.g. the amount of polyfunctional cells (cells producing 2 or more cytokines).
 Cells are first cultured on an antibody-coated surface, with or without the presence of your antigen of interest. During this culture, a selection of secreted cytokines are captured by antibodies bound to the culture surface. After a pre-specified period of stimulation, the labelled detection antibody will visualize the captured cytokines either through an enzymatic reaction (for singular detection) or a fluorescent label (when detection of multiple products is desired).
Cells are first cultured on an antibody-coated surface, with or without the presence of your antigen of interest. During this culture, a selection of secreted cytokines are captured by antibodies bound to the culture surface. After a pre-specified period of stimulation, the labelled detection antibody will visualize the captured cytokines either through an enzymatic reaction (for singular detection) or a fluorescent label (when detection of multiple products is desired).
Advantages of using an ELIspot for cytokine production detection
- High-throughput
- Robust and accurate (ideal for clinical testing)
- Up to three cytokines in a single test (with Fluorospot)
Example of cytokine detection via ELIspot
Assessing the amount of antigen-specific T cells in a PBMC sample
 PBMC were thawed and left to rest overnight. Subsequently, the cells were stimulated for 16 hours with a CEFT peptide pool containing CMV, EBV, Flu and Tetanus toxoid peptides. Afterwards, IFN-γ production was assessed via ELIspot. Left, representative images for unstimulated and CEFT-stimulated wells, right, compilation data of IFN-γ producing cells from 2 donors upon increasing concentrations of CEFT (3 replicates per condition, mean count ± standard deviation).
PBMC were thawed and left to rest overnight. Subsequently, the cells were stimulated for 16 hours with a CEFT peptide pool containing CMV, EBV, Flu and Tetanus toxoid peptides. Afterwards, IFN-γ production was assessed via ELIspot. Left, representative images for unstimulated and CEFT-stimulated wells, right, compilation data of IFN-γ producing cells from 2 donors upon increasing concentrations of CEFT (3 replicates per condition, mean count ± standard deviation).
Assessing SARS-CoV-2 cellular immunity via T cell ELISpot

PBMCs were isolated from healthy donors (HD, n=2) or ex-COVID patients (n=4) and, after cryopreservation, cells thawed and left to rest overnight. Subsequently, the cells were stimulated for 16 hours with a CEFT peptide pool containing CMV, EBV, Flu and Tetanus toxoid peptides, or SARS-CoV-2 nucleocapsid or spike (in 2 pools) peptides in triplicate. Afterwards, IFN-γ production was assessed via ELIspot. Left, representative images for unstimulated SARS-CoV-2, and CEFT-stimulated wells, right, compilation data of IFN-γ producing cells (3 replicates per condition, mean count over background). Dashed line indicates 2-fold change over background.
Multiplex assessment of T cell effector function with FluoroSpot
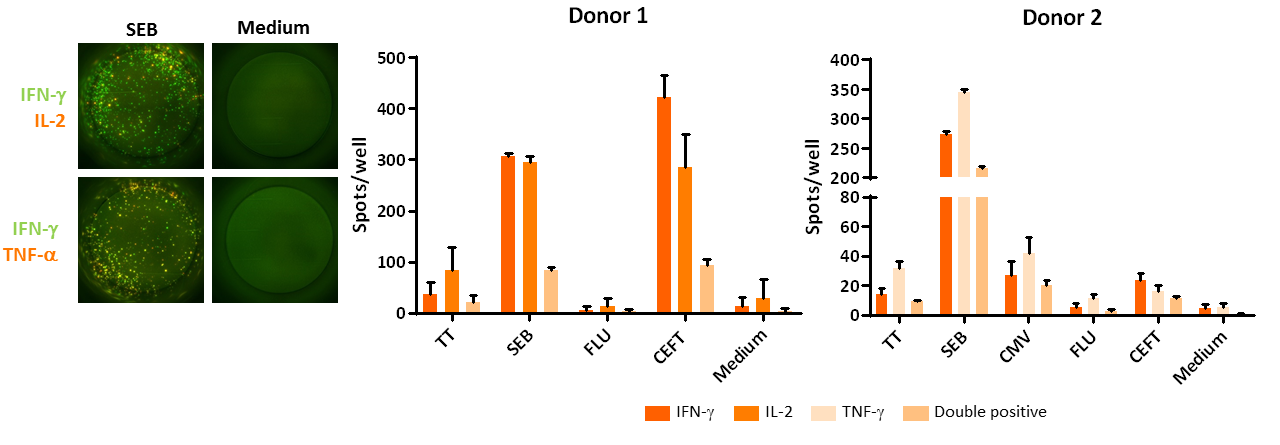
PBMCs were isolated from 2 healthy donors and, after cryopreservation, cells thawed and left to rest overnight. Subsequently, the cells were stimulated for 16 hours with tetanus toxoid (TT), Staphylococcal Enterotoxin B (SEB), influenza peptides (FLU) or CEFT (a peptide pool containing CMV, EBV, Flu and Tetanus toxoid peptides) in triplicate. Afterwards, IFN-γ, TNF-a and/or IL-2 production was assessed via FluoroSpot. Left, representative images for SEB-stimulated, or unstimulated wells, right, compilation data of cytokine producing cells (3 replicates per condition, mean count ± standard deviation).
For more applications of cytokine production analysis with ELIspot, see the reference below.
Reference
Monitoring T-Cell Responses in Translational Studies: Optimization of Dye-Based Proliferation Assay for Evaluation of Antigen-Specific Responses
ten Brinke A, Marek-Trzonkowska N, Mansilla MJ, Turksma AW, Piekarska K, Iwaszkiewicz-Grześ D, Passerini L, Locafaro G, Puñet-Ortiz J, van Ham SM, Hernandez-Fuentes MP, Martínez-Cáceres EM, Gregori S.
Front Immunol 2017;8:1870. doi: 10.3389/fimmu.2017.01870.

